Why the Eclipse System for Bowel Control?
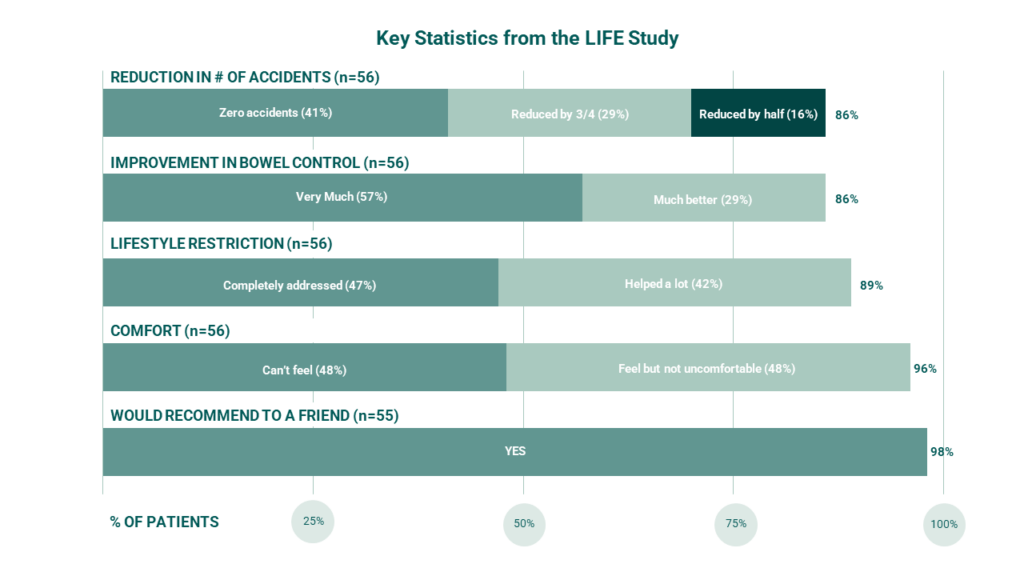
Immediate Results: In the LIFE Study, 8 of 10 women who were successfully fit with the Eclipse System reported treatment success during 1-month of use.6
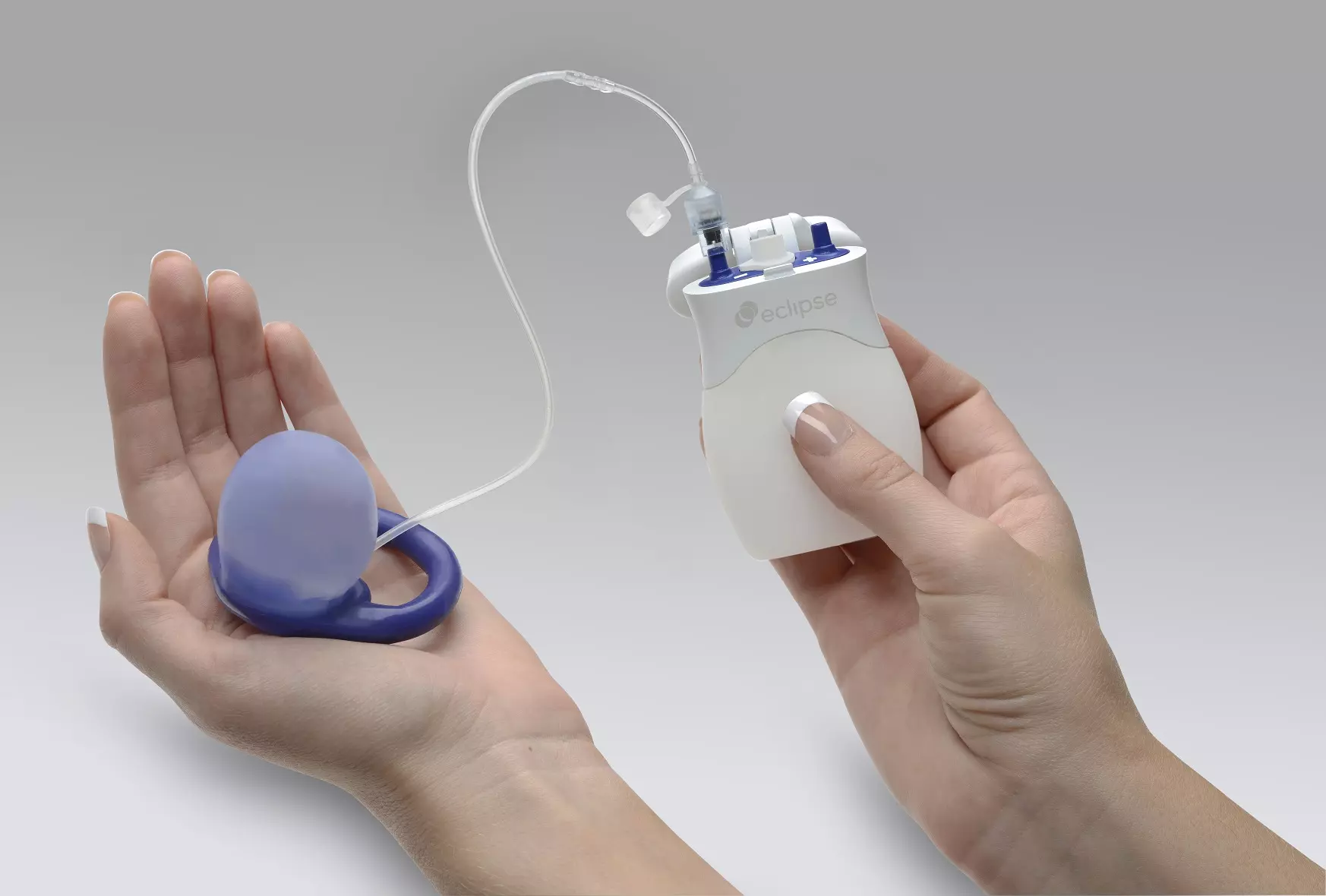
No Surgery Required: The Eclipse System is fit and provided in a physician’s office
Easy to Use: The vaginal insert is easy to place and easy to remove. Women can insert and remove the Eclipse System whenever they want.
What is Loss of Bowel Control?
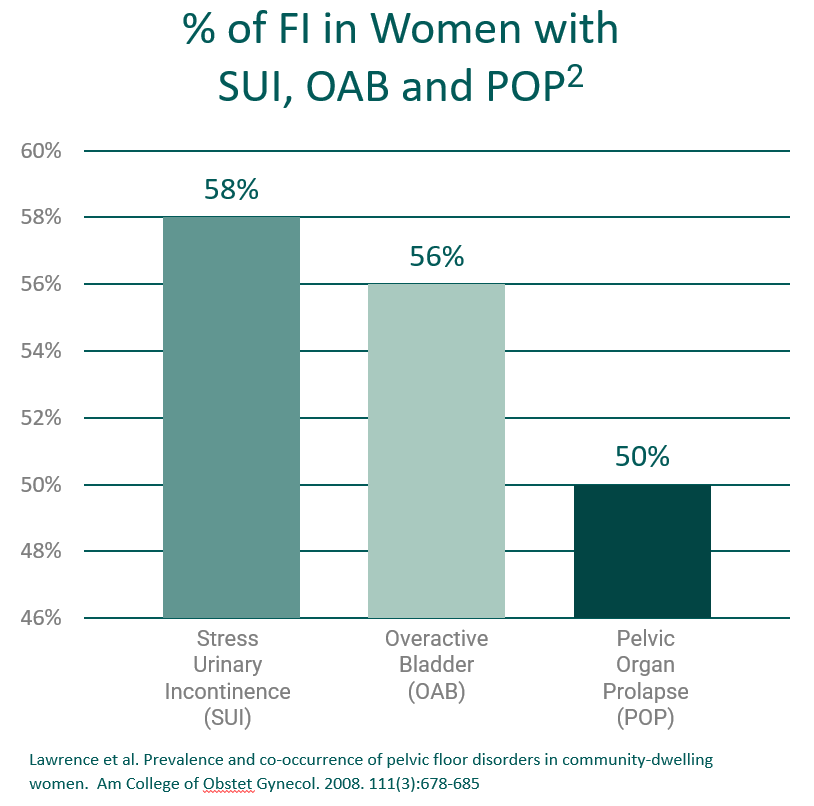
The inability to control one’s bowels which results in the leakage of stool – also known as Accidental Bowel Leakage (ABL) or Fecal Incontinence (FI) – is a real medical condition, and not merely a symptom of aging. Nerve or muscle damage in the pelvic region can affect bowel control, as can diarrhea, Irritable Bowel Syndrome or other gastrointestinal conditions. It can be embarrassing, even devastating in the wrong circumstances. Women who lose control of their bowels often feel like they have lost control of their life.
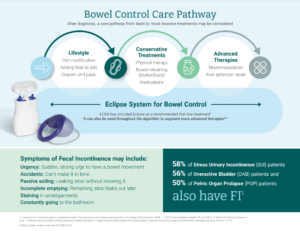
What are the Symptoms?
Loss of bowel control symptoms are varied. Common symptoms include:
- Urgency: Sudden, strong urge to have a bowel movement
- Passive soiling: Passing stool without knowing it
- Incomplete emptying: “Can’t get it all out” during a bowel movement; the remaining stool can leak out later on
Accidents can range from large to small to just stains in your underwear. Sufferers may have some good days and some bad days, or may have accidents every day. Women with bowel control issues may visit the bathroom frequently, and many report fearing a simple trip to work or the grocery store, and missing out on valuable time with friends and family. Unfortunately, many don’t bring it up with a doctor.